A5: Lanthanide iodides LnI_3: useful precursors for the synthesis of organometallic lanthanide complexes
The synthesis of organometallic lanthanide complexes presupposes useful precursors, which interact with different ligands to form stable compounds. Reactions like salt metathesis or acid-base reactions are common methods for creating these species. Varying functionalized precursors were established already, that suit differently in following reactions. Famous examples, useful in acid-base reactions are amides and borohydrides, while lanthanide halides suit best in salt metathesis. The importance of lanthanide halides, especially iodides, leads us to describe the synthesis of these precursors in detail on the example of LaI3.
In our case, we used La2O3 as starting material for the synthesis. La2O3 is an air-stable, cheap and commercially available solid. The procedure for the synthesis of LaI3 was usually conducted like it is described in the following section:[1]
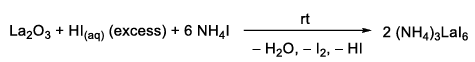
After using one equivalent of lanthanum oxide into a Schlenk flask, a 57% aqueous solution of hydroiodic acid (9 mL per mmoL La2O3) is added carefully. Directly, an exothermic reaction starts, which expresses with heat. The mixture is stirred for two hours until a clear solution without remaining solid is observed. Afterwards, Ar atmosphere is applied to the Schlenk flask and every further step of the synthesis is conducted under Schlenk conditions. Then 6.6 equivalents of NH4I are added and the mixture is stirred for 2 h at ambient temperature. After the reaction is complete, the solvent is removed under reduced pressure, which causes a colorless solid to precipitate. The colorless (NH4)3LaI6 is further dried (80°C, 24 h) to get rid of the remaining volatiles like HI and I2. As soon as the (NH4)3LaI6 is nearly colorless, it is transferred into one side of a glass pipe. Then, glass wool is set in the middle of the pipe, to separate the solid from the second part. Now the part with the (NH4)3LaI6 is placed into an oven (see Figure 1). The glass pipe is evacuated (~10-3 mbar) and the solid is heated continuously at 120°C for 6 h, then at 240°C for 24 h and finally at 360°C for 16 h.

After cooling to room temperature, the clean LaI3 (off-white powder on the right side of the glass tube) can be used directly in following reactions. All impurities have then been sublimated trough the glass wool to the left side of the glass tube (yellow solid), which is mainly ammonium iodide. By putting the tube into the glovebox and cracking it in half, the LaI3 can be collected. Average yields of this reaction are typically around 89%.

The LaI3 is soluble in THF and ether at room temperature as well as in hot toluene. We were able to use it in different salt metathesis reaction for instance with the potassium salts of a carbazolyl ligand and could stabilize solvent free LaIII complexes from toluene solution. Compared to other La halides like LaCl3, one big advantage of LaI3 is the solubility in aromatic solvents, which ensures a wide range of reactions. Both the properties of the LaI3 and its reactivity make it an important precursor for our research in the field of f-element complexes.
The above described synthetic pathway to lanthanide iodides does work for the synthesis of DyI3 and other trivalent lanthanides as well.
References
[1] G. Meyer, P. Ax, Mat. Res. Bull. 1982, 17, 1447-1455.

