C2: Isotopic labeling - possibilities and limitations
Isotopes are variants of a particular chemical element that have the same number of protons but different numbers of neutrons. This difference in neutron count results in varying atomic masses for the isotopes of an element, while their chemical properties remain mostly the same due to the unchanged proton and electron number. Hydrogen is the lightest element and its isotope protium consists of only one proton, one electron, and an atomic mass of roughly 1 au. The next heavier isotope of hydrogen is called deuterium and weighs roughly 2 au due to the additional neutron. Lastly, tritium, the lightest radioactive isotope, adds another neutron, resulting in a mass of approximately 3 au. [1,2]

As one can see, the proportionate mass difference between an element’s isotope is more pronounced in the elements with a low atomic number. Alongside the elements with different naturally occurring isotopes are elements with only one natural isotope like 19F, 23N, 31P, 127I and many more. Labeling these elements requires scientists to use radioactive isotopes. Analyzing different isotopes involves a range of techniques adjusted to the properties and applications of the isotopes in question. The most common techniques are mass spectrometry, nuclear magnetic resonance, and the detection of the specific type of radiation emitted by the radioactive isotopes. [2]
When talking about isotopes, one can differentiate between stable and radioactive isotopes. While the latter play a big part in medical diagnostics and treatment (e. g., 18F, 60Co, 99mTc, 131I, and 153Gd), they won’t be further discussed in this review due to their safety and regulatory concerns. [3–6]
There are generally two ways to obtain pure or enriched isotopes of one element – separation of different naturally occurring isotopes in a sample or manufacturing an isotope by irradiation. The separation relies on small differences in atomic weight, chemical reaction rate or properties like the nuclear resonance in so-called enrichment cascades. At the same time, the irradiation method utilizes high-energy radiation and a suitable target to produce the desired isotope. Since the separation of the isotopes is time and cost-intensive, labeled or enriched chemicals are often much more expensive than their unlabeled counterparts. [7,8]
Table 1: Approximate prices of isotopically labeled reagents for chemical synthesis. The cheapest prices for the respective purity are shown in € and were taken from Merck, Fisher Scientific, Carl Roth or the Cambridge Isotope Laboratories.
The price increase compared to the unlabeled chemical is even more pronounced for more complex molecules and unstable isotopes, making research with specific isotopes a huge financial burden. Another aspect to consider is that reactions and reagents with high atom economies are needed, to not waste valuable labeled elements. Therefore, often used standard reagents, like dimethyl sulfate as a methylating agent, can’t be used for isotopic labeling. The most common reagents for isotopic labeling are small and easy to prepare molecules that can be used in many ways like water (either D2O or H2 17/18O), 13C- methyl iodide and 15N-ammonium chloride.
One way to utilize different isotopes and their varying properties is isotopic labeling. This technique is prevalently used to track the passage of an isotope through a reaction, metabolic pathway, or a biological system via the exchange of the naturally occurring isotope with an isotope exhibiting the desired properties like nuclei spin (e. g. 15N or 17O). Another way to utilize isotopic labeling is by altering the vibrational energy of bonds. Due to the roughly doubled mass of deuterium compared to protium, the oscillation energy decreases. This effect is used in lanthanide luminescence to reduce the multiphonon relaxation and, thus, to increase the emission. These differences in properties are called isotope effect.
The most used isotopes are 2H, 13C, 15N, 17O and 18O. To incorporate these into modern synthesis, scientists developed numerous routes from simple substitutions to biosynthetic procedures involving microorganisms or plants. The following sections show some methods for each isotope in more detail.
Deuterium-labeling
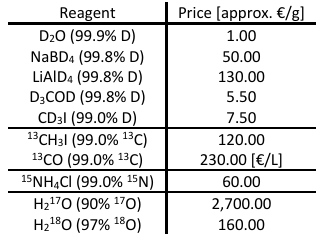
Deuteration of molecules can be done in multiple ways, depending on the molecule in question. The easiest way is a simple substitution of hydrogen in deuterated solvents like D2O, either with or without an additional base. [9] Another option is the deuteration of an olefine via D2 and a precious metal catalyst like platinum. [1,10] Lastly, deuterated reducing agents like NaBD4 or LiAlD4 can be used to reduce, for example, carbonyls to hydroxyl-groups, leaving the product with deuterium instead of hydrogen. [9]
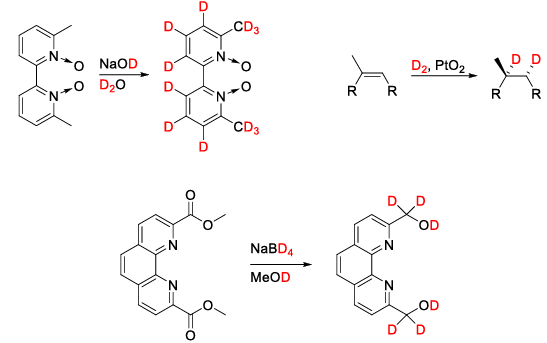
Carbon-labeling
The easiest way to introduce carbon-labeling into a molecule is with 13C-methyl iodide. Because of its relatively low price, wide range of possible substrates and reaction types like methylation and Grignard-reactions 13CH3I became the go-to 13C-labeling reagent for synthetic chemists. [11] In a biochemistry setting it is possible to obtain labeled compounds via the 13CO2 metabolism of plants and other biological systems. [12]
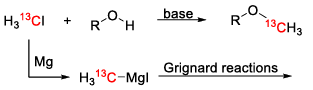
Nitrogen-labeling
Nitrogen-labeling is often used in a biochemistry setting to understand more about the pathways in a biological system. Therefore, many amino acids and proteins were labeled using simple 15N based molecules like ammonium chloride or ammonia. [13] Another good starting point are 15N-labeled amines with their various reaction types, like acylations.
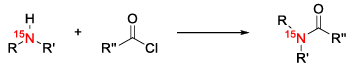
Oxygen-labeling
Oxygen has two stable isotopes suitable for isotopic labeling – 17O and 18O. The most common reagent for the incorporation of a specific oxygen isotope is labeled water. One such example is the oxygen transfer published by Rozen et. al. via hypofluorous acid. The procedure allows the oxidation of carbon atoms next to carbonyl groups or heterocycles like phenanthroline. The required HOF is generated by the reaction of fluorine with water. [14,15] More commonly used oxidation methods from ordinary synthetic chemistry like peroxy acids aren’t used in isotopic labeling because of their poor atom efficiency regarding their labeled oxygen isotopes.
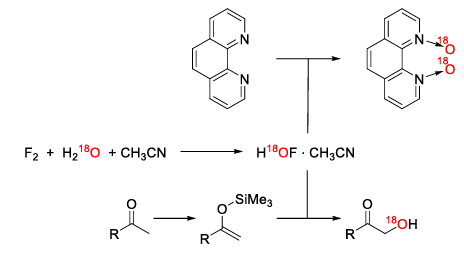
As one can see, isotopic labeling is a powerful and versatile tool in scientific research, enabling detailed studies of reaction pathways and in medical applications. However, the technique comes with significant costs, driven by the need for enriched isotopes, complex synthetic procedures, and stringent safety measures. Understanding the economic and synthetic aspects of isotopic labeling is crucial for optimizing its use in various applications. As technology advances continue to reduce costs and improve synthetic methodologies, isotopic labeling will become even more accessible and impactful in scientific research.
References
[1] E. Riedel, C. Janiak, Anorganische Chemie, Walter De Gryter GmbH, Berlin/Boston, 2015.
[2] G. Faure, T. M. Mensing, Isotopes - Principles and Applications, John Wiley & Sons, Inc., Hoboken, New Jersey, 2005.
[3] H. Jadvar, P. M. Colletti, Eur. J. Radiol. 2014, 83, 84–94.
[4] P. Mayles, A. Nahum, J. Rosenwald, Handbook of Radiotherapy Physics, Taylor & Francis Group, LLC, Abingdon-on-Thames, 2007.
[5] J. R. Dilworth, S. J. Parrott, Chem. Soc. Rev. 1998, 27, 43–55.
[6] U. Mallick, C. Harmer, B. Yap, N. Engl. J. Med. 2012, 366, 1675–1685.
[7] A. Kohen, H.-H. Limbach, Isotope Effects in Chemistry and Biology, Taylor & Francis, Boca Raton, 2006.
[8] S. M. Qaim, Radiochim. Acta 2012, 100, 635–651.
[9] C. Bischof, J. Wahsner, J. Scholten, S. Trosien, M. Seitz, J. Am. Chem. Soc. 2010, 132, 14334–14335.
[10] M. Oba, T. Terauchi, A. Miyakawa, H. Kamo, Tetrahedron Lett. 1998, 39, 1595–1598.
[11] G. Sulikowski, M. Sulikowski, M. Haukaas, B. Moon, in Encyclopedia of Reagents for Organic Synthesis, 2005.
[12] Z. Li, Q. Yao, X. Guo, Front. Microbiol. 2019, 10, 1–14.
[13] H. Yu, P. Chaimbault, I. Clarot, Z. Chen, P. Leroy, Talanta 2019, 191, 491–503.
[14] S. Dayan, Y. Bareket, S. Rozen, Tetrahedron 1999, 55, 3657–3664.
[15] S. Rozen, S. Dayan, Angew. Chem. Int. Ed. 1999, 38, 3472–3473.

