A4: Synthesis of rare-earth nanoparticles
Knowledge on zerovalent rare-earth metal nanoparticles has been extremely limited in literature so far. This is primarily attributable to the extremely high reactivity of rare-earth metal nanoparticles when in contact with air, moisture or other oxidizing reagents, which requires the particles to be synthesized and handled under strictly inert conditions. This high reactivity of is caused by the combination of the low electrochemical potentials of the rare earth metals (about −2.4 V)[1], the high surface area of the respective nanoparticles and the absence of a passivation layer formed by rare-earth oxides, hydroxides or carbonales. Therefore, it is not surprising that the synthesis of rare-earth metal nanoparticles has been almost exclusively limited to physical methods such as laser ablation or vapor condensation, which could only yield highly agglomerated particles with a broad size distribution.[2,3] On the other hand, liquid phase syntheses for zerovalent rare earth nanoparticles have been practically unknown. In literature, only the reduction of GdCl3 with alkalides in tetrahydrofuran (THF) for the preparation of Gd-nanoparticles has jet been described. However, due to the high reactivity of the obtained Gd(0) nanoparticles, the authors could only identify Gd2O3 nanoparticles via electron microscopy.[4]
2021, the Feldmann group successfully developed a liquid-phase synthetic approach for the preparation of high-quality, oxide-free rare earth metal nanoparticles with narrow size distributions starting from simple, commercially available rare-earth metal halides. In this synthetic approach, the respective rare earth metal halide is reduced in ethers like THF using lithium naphthalenide ([LiNaph]) as the reducing agent to obtain the zerovalent rare-earth metal nanoparticles. For this purpose, the rare-earth metal halide as well as lithium and naphthalene are dissolved separately in THF. Thereafter, the resulting [LiNaph] solution is injected with strong stirring into solution of the metal halide. Here, the successful reduction of the metal halide and nucleation of nanoparticles is indicated by the formation of a deep black suspension after injection of [LiNaph] (Figure 1).[5]
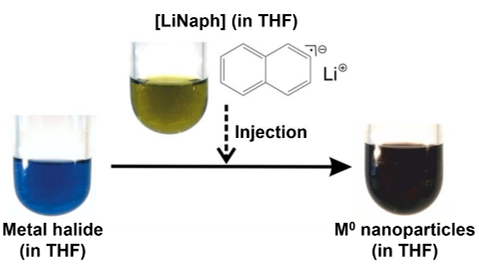
However, it must be noted that not all rare earth metal halides have sufficient solubility in THF to prepare a solution with a reasonable amount of solvent. To be able to prepare nanoparticles from these rare earths nonetheless, the synthetic strategy just presented can be modified as a one-pot approach, where the rare-earth metal halide, lithium and naphthalene are reacted in THF for 12 hours. In this one-pot approach, first, [LiNaph] is formed from the reaction of the lithium metal with the naphthalene. Moreover, the rare-earth metal halide is slowly dissolved into the THF and instantaneously reacts with [LiNaph] to form rare-earth metal nanoparticles (Figure 2).[6]
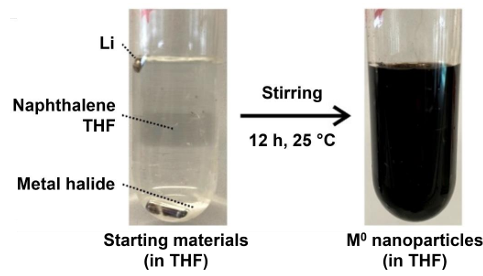
Regardless of which of the two synthetic approaches was used, the resulting rare earth nanoparticles are purified by repeated centrifugation and redispersion in/from THF or toluene to remove residual starting material, naphthalene and LiCl. Thereafter, the rare-earth metal nanoparticles can be either redispersed in THF or toluene to obtain long-term stable suspensions or dried in vacuum to obtain them in powdered form.
References
[1] A. F. Holleman, E. Wiberg, Anorganische Chemie, 103. ed., de Gruyter, Berlin, 2017.
[2] N. V. Tarasenko, A. V. Butsen, A. A. Nevar, Appl. Phys. A 2008, 93, 837-841.
[3] V. I. Petinov, Russ. J.Phys. Chem. A 2016, 90, 1413-1418.
[4] J. A. Nelson, L. H. Bennett, M. J. Wagner, J. Am. Chem. Soc. 2002, 124, 2979-2983.
[5] D. Bartenbach, O. Wenzel, R. Popescu, L.-P. Faden, A. Reiß, M. Kaiser, A Zimina, J.-D. Grunwaldt, D. Gerthsen, C. Feldmann, Angew. Chem. Int. Ed. 2021, 60, 17373-17377.
[6] A. Reiß, S. Schlittenhardt, M. Ruben, C. Feldmann, Z. Anorg. Allg. Chem. 2022, 648, e202200299.

