B1: Dinuclear 3d-4f single molecule magnets messenger tagging spectroscopy
Introduction
Single molecule magnets (SMMs) are a unique class of coordination compounds that exhibit magnetic bistability at the molecular level. These materials have garnered significant interest due to their potential applications in data storage, quantum computing, and molecular spintronics. Within this class of compounds mixed 3d-4f SMMs are of high interest as the combination of 3d and 4f ions often leads to intriguing magnetic behaviours which are not observed in purely 3d- or 4f-based SMMs. In order to get insights into the interplay between the different electronic properties of the two sorts of metal ions, dinuclear 3d-4f SMMs seem to be very promising as they allow for a combination of experimental measurements with theoretical calculations.[1-4]
Structural Characteristics
Dinuclear 3d-4f SMMs feature exactly one central transition metal ion and one lanthanide ion, each coordinated by one or more ligands bridging the two metal centres. These ligands can facilitate magnetic coupling between the 3d and 4f centres. Often additional co-ligands are present in order to complete to coordination spheres of the respective metal ions. The coordination environments and the nature of the ligands play crucial roles for the resulting magnetic properties of these complexes. Structures are commonly characterized using X-ray crystallography, which provides detailed information about the coordination environment and the distances between the metal centres.
The main challenge for designing dinuclear 3d-4f complexes is the different coordinating properties exhibited by 3d and 4f metal ions. According to the hard and soft acids and bases (HSAB) concept, harder donating ligands will tend to coordinate only to 4f ions, whereas softer donating ligands will tend to coordinate only to softer 3d ions. So, in order to prevent the formation of compounds with only one sort of metal ion or clusters with higher nuclearities, often special types of bridging ligands need to be used. So-called “compartmental ligands” have different coordination pockets with selective binding properties towards either 3d or 4f metal ions. They are able to coordinate and link the different types of metal ions by capturing them in their favoured coordination pockets. Common bridging ligands include oxygen- and nitrogen-donor atoms, whereas additional co-ligands used to complete the coordination sphere of the lanthanide ion, are usually hard oxygen-based donors such as nitrates or carboxylates. A schematic representation of a typical dinuclear 3d-4f system is presented in Figure 1.[4-6]
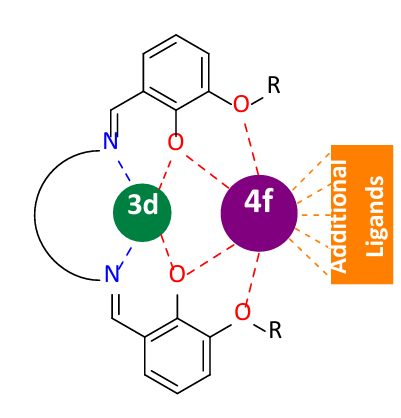
Synthesis
The synthesis of dinuclear 3d-4f SMMs involves the careful selection of ligands and metal precursors to achieve the desired coordination environment and magnetic properties. Typical synthetic strategies include:
1. Direct Combination: Mixing solutions of 3d and 4f metal salts with appropriate ligands under controlled conditions to form the dinuclear complex.
2. Stepwise Assembly: Sequentially introducing the 3d and 4f metal ions to a pre-formed ligand scaffold, allowing for a better control over the final structure.
3. Solvothermal Methods: Using high temperature and pressure conditions to promote the formation of the desired dinuclear complex, sometimes resulting in higher crystallinity and purity.[5-6]
Magnetic Properties
One of the key features of SMMs is their ability to exhibit slow relaxation of magnetization, a phenomenon where the magnetic moment of the molecule retains its orientation over extended periods, even in the absence of an external magnetic field. This behaviour is often quantified by the energy barrier for magnetization reversal (ΔU), which depends on the magnetic anisotropy and the coupling between the metal ions.
The magnetic properties of 3d-4f SMMs are a result of the interplay between the spin states of the 3d transition metal ions and the 4f lanthanide ions. The idea for using dinuclear 3d-4f compounds as SMMs is to combine the anisotropic nature and the large magnetic moments of 4f ions with slow relaxation rate and diverse coordination chemistry known for 3d ions in order to obtain large energy barriers and suppressed relaxation. Moreover, calculating electronic states and magnetic interactions of compounds with up to two metal centres is possible with recent progress in the development of calculation programs. Dinuclear complexes are therefore particularly informative allowing a combination of these quantum mechanical ab initio calculations with experimental data from magnetic measurements.[7-9]
Potential Applications
The interesting magnetic properties of dinuclear 3d-4f SMMs make them promising candidates for various advanced technological applications:
1. Data Storage: The ability of SMMs to maintain their magnetic orientation at the molecular level makes them suitable for high-density data storage devices, where each molecule can represent a single bit of information.
2. Quantum Computing: The quantum superposition and entanglement properties of SMMs can be exploited in quantum computing for the development of qubits, the fundamental units of quantum information.
3. Spintronics: SMMs can be integrated into spintronic devices, where the spin of electrons, rather than their charge, is used to carry information, potentially leading to faster and more energy-efficient electronic devices.[9-10]
Challenges and Future Directions
Despite their promising properties, dinuclear 3d-4f SMMs face several challenges that must be addressed before they can be widely applied. These include improving the thermal stability and increasing the operating temperatures of these materials, as most SMMs exhibit their unique magnetic properties only at very low temperatures. Additionally, the development of scalable and cost-effective synthesis methods is crucial for practical applications.
Future research in this field is likely to focus on designing new ligands and synthetic strategies to tune the magnetic properties and stability of dinuclear 3d-4f SMMs. Advances in computational modelling and magnetic characterization techniques will play a critical role in understanding magnetic interactions within these compounds.
References
[1] R. Sessoli, D. Gatteschi, A. Caneschi, M. A. Novak, Nature 1993, 365, 141-143.
[2] G. Christou, D. Gatteschi, D. N. Hendrickson, R. Sessoli, MRS Bull. 2000, 25, 66-71.
[3] R. Sessoli, A. K. Powell, Coord. Chem. Rev. 2009, 253, 2328-2341.
[4] H. L. C. Feltham, S. Brooker, Coord. Chem. Rev. 2014, 276, 1-33.
[5] B. Monteiro, J. Coutinho, L. Pereira, Heterometallic 3d-4f SMMs. In Lanthanide-Based Multifunctional Materials, Vol.1, P. Martin-Ramos, M. R. Silva, Elsevier, Amsterdam, Oxford, 2018, pp. 233-261.
[6] M. Andruh, Chem. Commun. 2011, 47, 3025-3042.
[7] S. T. Liddle, J. van Slageren, Chem. Soc. Rev. 2015, 44, 6655-6669.
[8] J. D. Rinehart, J. R. Long, Chem. Sci. 2011, 2, 2078-2085.
[9] T. Bodenstein, K. Fink, Nachr. Chem. 2016, 65, 339-342.
[10] E. Moreno-Pineda, W. Wernsdorfer, Nat. Rev. Phys. 2021, 3, 645-659.

